On July 9, 2021, the research outcomes of a multi-center large-scale clinical study (RESOLVE) of locally advanced gastric cancer led by Chinese experts were online published in The Lancet Oncology (impact factor 41.316), a top-notched international oncology journal. The RESOLVE study entitled "Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial" was launched in 2012, which is a paradigm of multi-disciplinary and multi-center clinical trial in the field of gastric cancer in China. It sets up an exemplary for high-quality clinical trial for gastric cancer in terms of overall design of research project, participation of multi-disciplinary experts, multi-centers and financial support from enterprises,etc.
As a multi-center large-scale trial of locally advanced gastric cancer in China, the RESOLVE study has the following characteristics: Chinese population, high-risk gastric cancer, large sample size and prospective design. As one of the co-authors and affiliations of this article, the team led by Professor Dang Chengxue from Department of Oncology Surgery of the First Affiliated Hospital of Xi’an Jiaotong University (XJTU) started to participate in the RESOLVE study in 2014. As the only research affiliation in Northwest China, the team has completed the clinical trial with high quality and rigorous scientific attitude.

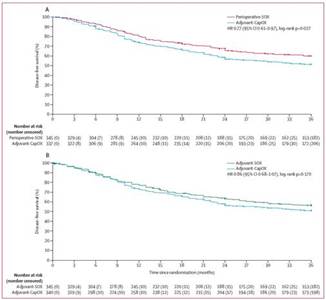
The RESOLVE study is a three-arm, randomised, multi-center, open-label phase III trial, aiming to compare the efficacy and safety of XELOX (capecitabine+oxaliplatin) (group A) or SOX (oxaliplatin+TS-1) (group B) after D2 gastrectomy and perioperative SOX (group C). The findings demonstrated that compared with CapOx regimen (capecitabine combined with oxaliplatin), perioperative and postoperative SOX regimen in patients with locally advanced gastric cancer undergoing standard D2 gastrectomy yielded clinically meaningful improvement in survival and significantly reduced the risk of 3-year recurrence or mortality by 23%. Meantime, SOX was non-inferior to CapOx regimen during postoperative adjuvant treatment for these patients. It is "the first large-scale randomised trial which directly compares neoadjuvant therapy with adjuvant therapy in locally advanced gastric cancer patients", and it is also "the first study to demonstrate that perioperative SOX chemotherapy significantly improves the 3-year disease-free survival compared with CapOx adjuvant therapy". The RESOLVE trial adds new evidence for the application of perioperative SOX for patients with locally advanced gastric cancer, bringing significant guiding value for optimizing perioperative therapeutic regime for gastric cancer.
In addition, Professor Dang Chengxue and his team will continue to participate in nationwide multi-center RESOLVE 2 trial in the post-RESOLVED era. This study is designed to compare the perioperative efficacy between DOS (docetaxel, oxaliplatin and S-1) and SOX (oxaliplatin andS-1), aiming to provide better solutions for gastric cancer patients in China.