On July 17, 2021, the research outcomes of an article entitled “Icotinib versus chemotherapy as adjuvant treatment for stage II-IIA EGFR-mutant non-small-cell lung cancer (EVIDENCE): a randomised, open-label, phase 3 trial”, jointly carried out by Professor Zhou Caicun from Shanghai Pulmonary Hospital affiliated to Tongji University and He Jianxing, President of the First Affiliated Hospital of Guangzhou Medical University, were published inThe Lancet Respiratory Medicine (impact factor: 30.143), a top-notched academic journal around the globe.

The research team led by Professor Fu Junke from Department of Thoracic Surgery of the First Affiliated Hospital of Xi’an Jiaotong University (XJTU) participated in this EVIDENCE clinical trial. As a major participant, Professor Fu Junke was listed one of the co-authors, and the research team from Department of Thoracic Surgery of our hospital also became one of the affiliations of this article. This study is a randomised, open-label, phase 3 trial, and it is also the first registered clinical trial in the field of postoperative adjuvant treatment for non-small cell lung cancer in China. A total of 29 medical centers across China participated in this 6-year clinical trial with an enrollment of 322 patients. Department of Thoracic Surgery of our hospital participated in this clinical trial on June 8, 2015, and 8 patients were enrolled, ranking 11thamong all research institutions. As the only research affiliation in Northwest China, Department of Thoracic Surgery of our hospital has completed the clinical trial with high quality and rigorous scientific attitude.
EVIDENCE clinical trial demonstrated that the median DFS was 47.0 months in the Icotinib adjuvant targeted therapy group and 22.1 months in the standard adjuvant chemotherapy group (HR=0.36, 95%CI: 0.24-0.55,P<0.0001). According to the clinical characteristics of each subgroup, DFS in the Icotinib adjuvant targeted therapy group was superior to that in the standard adjuvant chemotherapy group. The 3-year DFS rates in the Icotinib adjuvant targeted therapy group and standard adjuvant chemotherapy group were 63.88% and 32.47%, respectively. The 5-year DFS rate remains to be calculated. In terms of therapeutic safety, the incidence of adverse events in the Icotinib adjuvant targeted therapy group was significantly lower compared with that in the standard adjuvant chemotherapy group, and the incidence of grade 3 and above adverse events was 11% and 61%, respectively. In the Icotinib adjuvant targeted therapy group, rash was the common adverse event, whereas gastrointestinal reactions, such as nausea and vomiting and hematological toxicity were mainly observed in the standard adjuvant chemotherapy group.
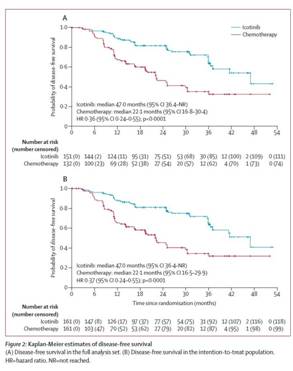
This EVIDENCE clinical trial confirms that Icotinib yields higher clinical efficacy in the treatment of EGFR-mutant non-small-cell lung cancercompared with standard adjuvant chemotherapy. Moreover, postoperative adjuvant targeted therapy significantly prolongs the DFS of patientsand elevates the therapeutic safety. Lcotinib is a novel therapeutic option for EGFR-mutant non-small-cell lung cancerpatients, which yields high efficacy and low toxicity.