Recently, Cell Press, publisher of the world-leading international academic journal Cell, announced the 2024 “Most Popular China Paper Award”. The team of Yuan Zuyi, Wu Yue and Li Ting from the First Affiliated Hospital (FAH) of Xi’an Jiaotong University (XJTU) received this honor for their paper entitled “A gut microbiota-bile acid axis promotes intestinal homeostasis upon aspirin-mediated damage” published in Cell Host & Microbe.

The selection for this award covered five major fields: life sciences, physical sciences, medicine, interdisciplinary sciences and sustainable development. Based on nominations from journal editorial offices and evaluation by third-party experts, and taking into account multiple indicators such as article download counts, citation frequency and degree of innovation, a total of 50 “Papers of the Year” and 25 “Most Popular Papers” were ultimately selected.

The findings were published in Cell Host & Microbe, a microbiology subjournal of the journal Cell. The paper systematically elucidates a novel mechanism by which aspirin induces intestinal injury and, for the first time, proposes an intervention strategy targeting the “gut microbiota-bile acid axis”, providing an entirely new approach for the prevention and treatment of adverse effects associated with aspirin and other cardiovascular drugs.
This study was jointly completed by Professors Yuan Zuyi and Wu Yue from the FAH of XJTU in collaboration with Professor Frank J. Gonzalez from the U.S. National Institute of Medicine. By integrating multi-omics data from clinical cohorts with animal trials, the team, for the first time, revealed the key protective role of the “gut microbiota-bile acid axis” in aspirin-related intestinal injury, and identified Parabacteroides goldsteinii (P. distasonis), which is inhibited by aspirin, and its metabolite 7-keto-lithocholic acid (7-keto-LCA). The latter promotes intestinal stem cell renewal and epithelial repair by inhibiting FXR signaling and activating Wnt signaling. Supplementation with P. distasonis alleviated intestinal barrier injury in mice, providing a new microbial-metabolic intervention strategy for the prevention and treatment of aspirin-related enteropathy.

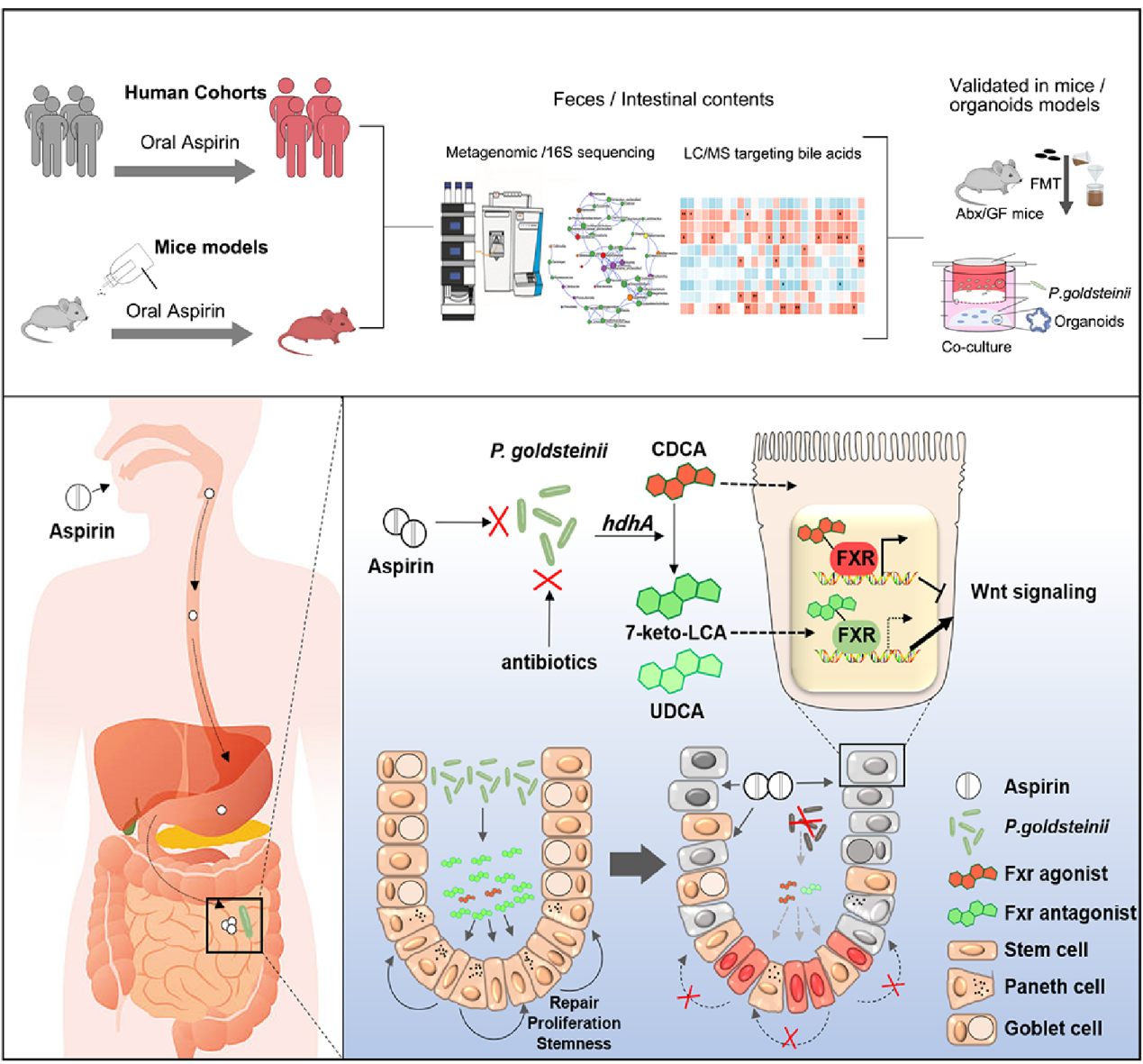
The research findings have deepened the understanding of the mechanisms underlying the intestinal adverse effects of aspirin and have provided an important theoretical basis for the development of individualized gastrointestinal protective strategies based on the microbiome and metabolome. The research team stated that they will further carry out translational clinical research in the future to explore feasible approaches for preventing and treating aspirin-related intestinal injury by modulating the gut microbiota, and to promote the application of “precision microecological therapy” in clinical pharmacy.
Article link: https://www.cell.com/cell-host-microbe/fulltext/S1931-3128(23)00510-3