On July 28, 2023, Professor Wang Qiang, discipline leader of anesthesiology in the First Affiliated Hospital (FAH) of Xi 'an Jiaotong University (XJTU), and Professor Ma Daqing from Imperial College London jointly published a research article entitled "Reducing complement activation during sleep deprivation yields cognitive improvement by dexmedetomidine" in the British Journal of Anaesthesia (IF: 9.8 in 2023). This study, for the first time, reveals that dexmedetomidine (DEX) inhibits complement C3-C3aR activation and complement-mediated synaptic elimination during sleep deprivation, elucidates the new mechanism underlying the effect of DEX upon preventing sleep loss-related cognitive decline, and provides novel therapeutic strategies for sleep loss-related diseases.

DEX is commonly used for perioperative sedation in the intensive care unit (ICU) patients, which can effectively reduce the incidence of postoperative cognitive dysfunction, whereas whether DEX can improve cognitive deficits caused by sleep deprivation and its specific mechanism remain unknown. Consequently, the research team established mouse models of chronic sleep restriction (CSR), and found that mice with CSR showed a decline in learning and memory capability, while intravenous injection of DEX could mitigate cognitive dysfunction in CSR mice. EEG showed that DEX could significantly prolong the non-rapid eye movement (NREM) sleep duration in CSR mice and increase the proportion of delta waves during NREM sleep, fully suggesting that DEX can not only enhance sleep quality, but also alleviate cognitive deficits caused by sleep deprivation.
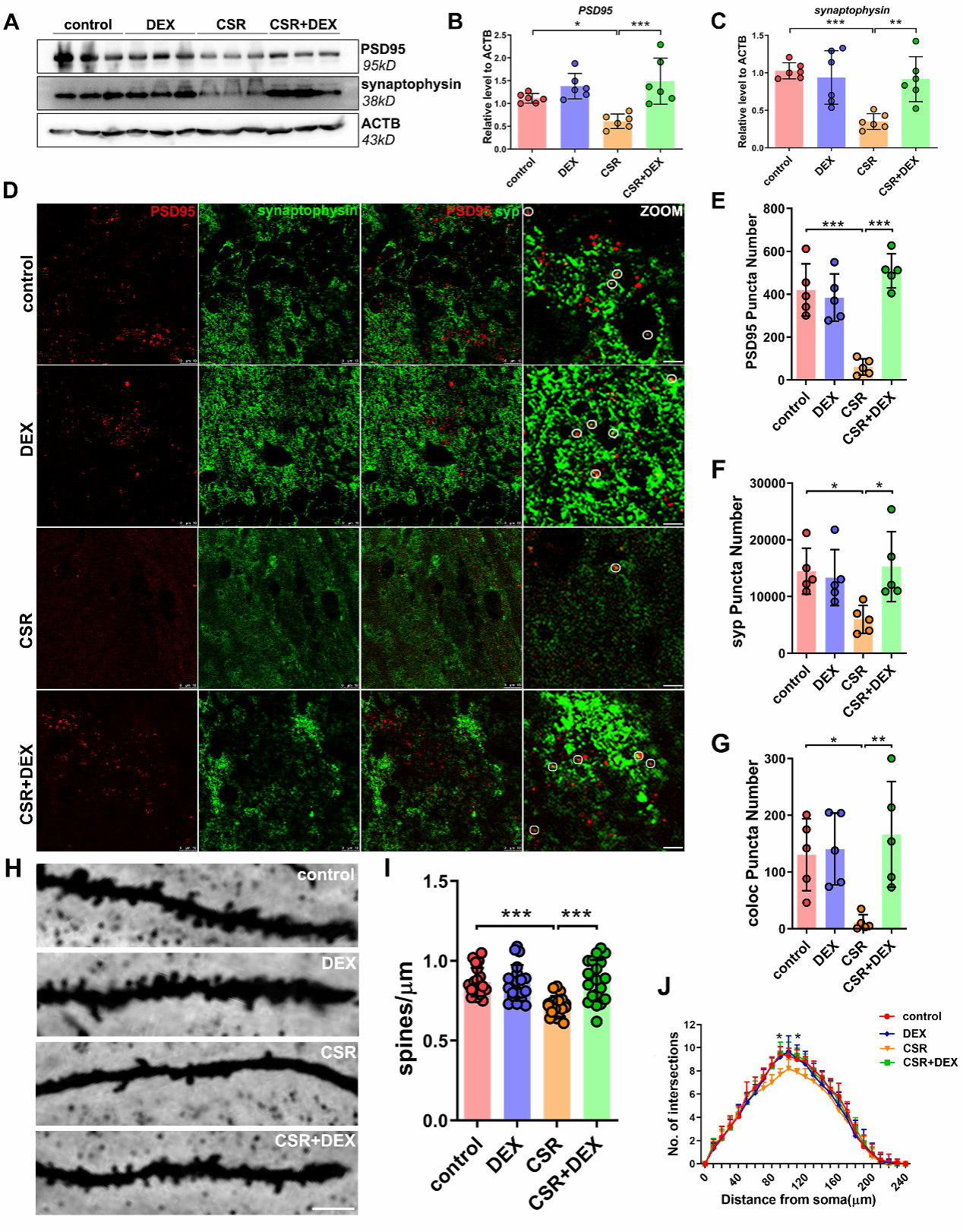
To further clarify the mechanism of DEX in improving cognitive dysfunction in mice with sleep deprivation, researchers found that the expression levels of synapse-related proteins PSD95 and synaptophysin were down-regulated in CSR mice by extracting hippocampus tissues in mice, while DEX could reverse the decreased expression levels of PSD95 and synaptophysin. Golgi-Cox staining also confirmed that DEX could reverse the decrease of dendritic spines.
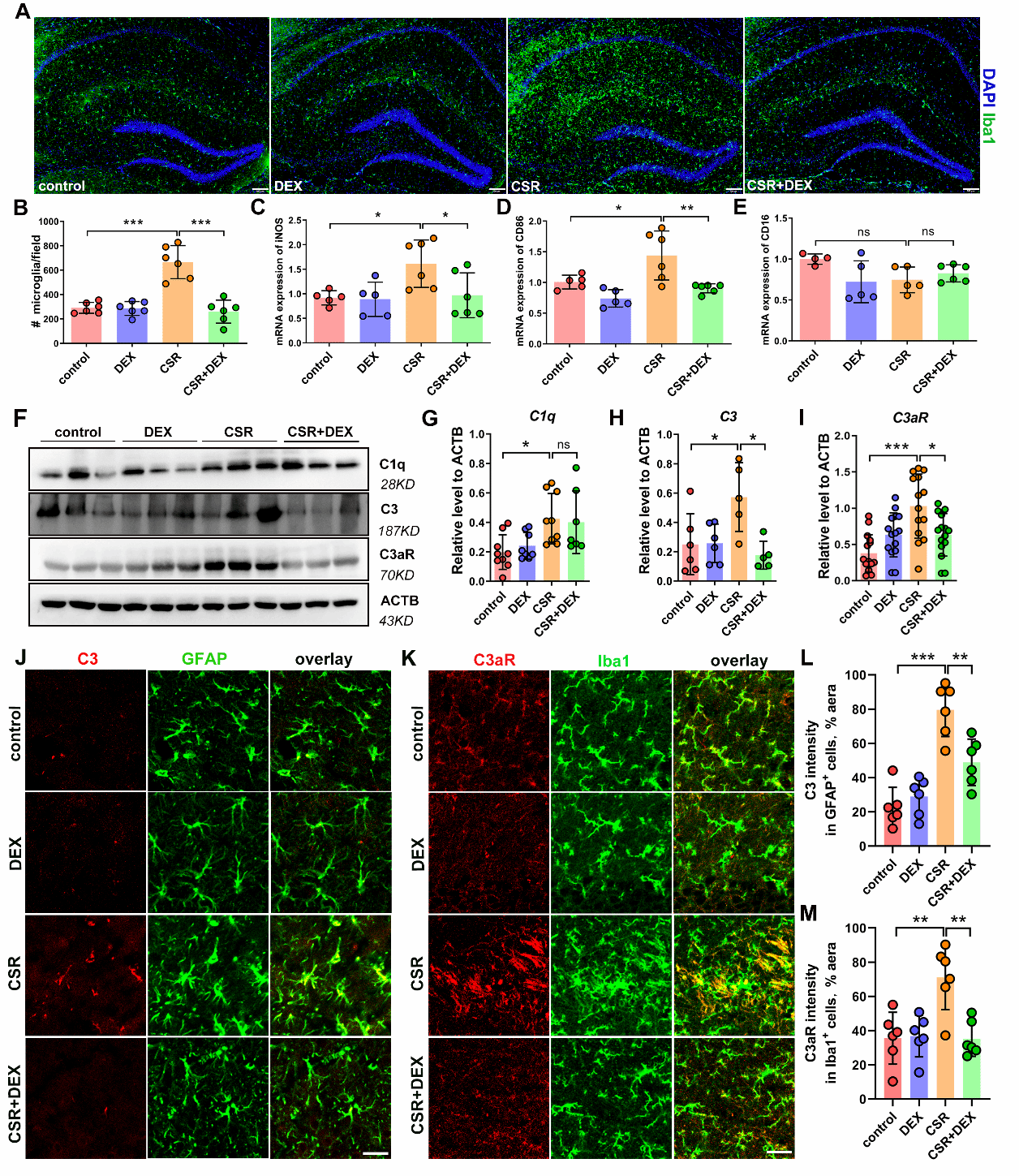
Hence, is the microglial synaptic elimination mediated by complement the cause of synaptic changes? The researchers found that microglia were activated after sleep deprivation, and the expression levels of complement C1q, C3 and C3aR were significantly up-regulated. DEX can inhibit the activation of microglia and the expression of complement C3 and C3aR, prompting that microglial synaptic elimination mediated by complement may be involved in the effect of DEX on improving sleep loss-related cognitive dysfunction.
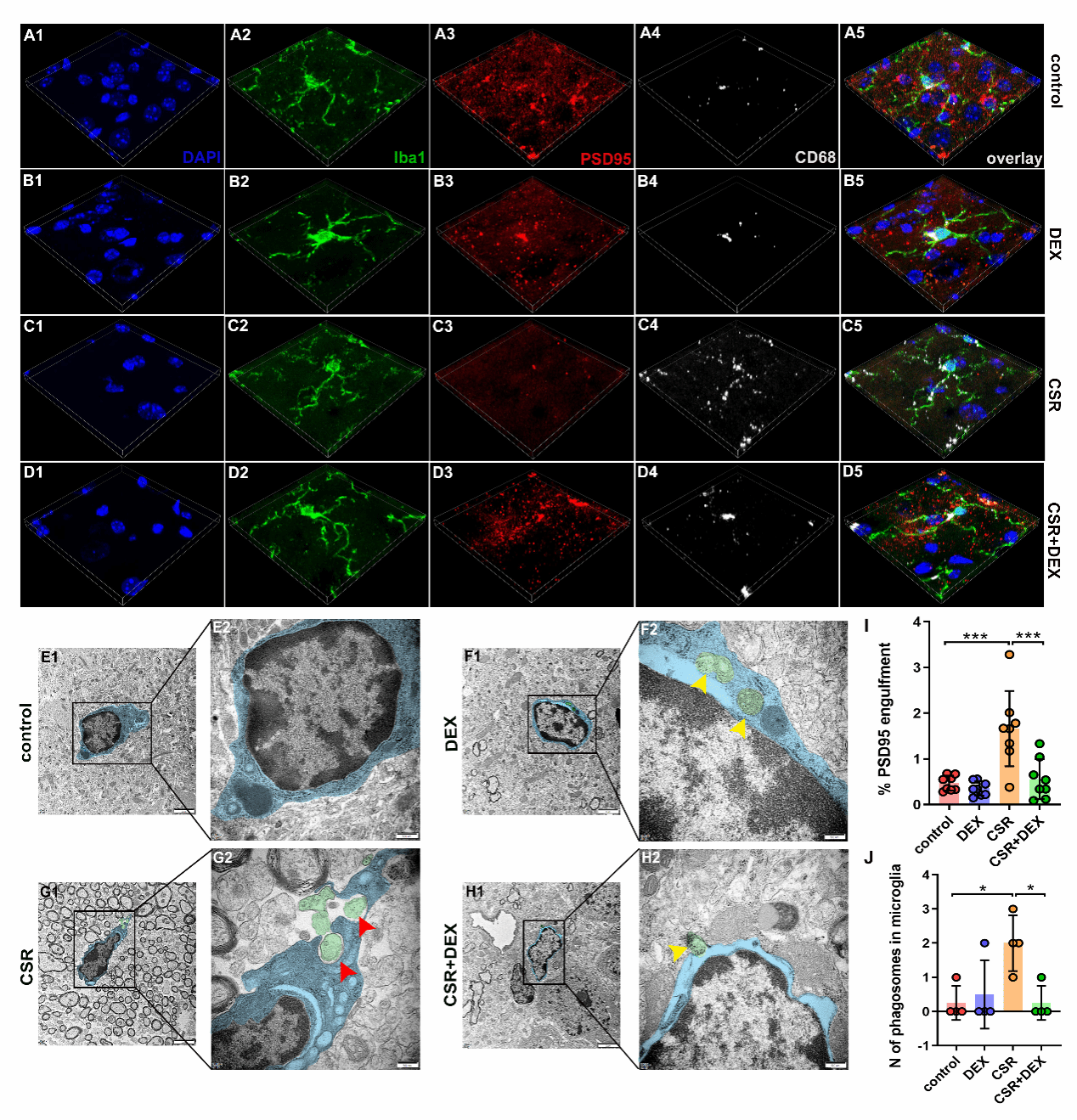
The researchers further observed the changes of microglial phagocytosis, and found that phagocytic microglia engulfed more synapses and lost more synapses in CSR mice. DEX inhibited microglial phagocytosis of synapses, which further confirmed that complement-mediated microglial synaptic elimination was a main cause of cognitive decline after sleep deprivation. DEX suppressed synaptic elimination process, thereby mitigating cognitive dysfunction.
Although DEX has been confirmed to inhibit the activation of complement signals, whether DEX can change cognitive function and the number of synapses in mice after blocking C3-C3aR signaling pathway remains elusive. The researchers treated the mice with C3aR antagonist SB290157 and found that blocking C3-C3aR signaling pathway could also mitigate cognitive decline and synaptic loss in CSR mice, which did not differ from the effect of DEX alone.
Finally, whether DEX protects against complement-mediated synaptic elimination process via activating α2 adrenoceptors in the brain is still unclear. α2 adrenoceptors mainly comprise α2A, α2B and α2C subtypes, among which α2A and α2C receptors are mainly distributed in the central nervous system. α2A adrenoceptor is mainly expressed in the hippocampus of mice, only expressed on the surface of astrocytes. Researchers micro-injected a2A adrenoceptor antagonist BRL-44408 into the brain in a stereotactic manner, and demonstrated that DEX could improve cognitive function, down-regulate the expression of complement C3 and C3aR, and inhibit microglial phagocytosis of synapses, indicating that DEX could mitigate cognitive deficits related to sleep deprivation by activating α2A adrenoceptors in astrocytes.This study, for the first time, reveals that DEX activates α2A adrenoceptors in astrocytes to inhibit its release of complement C3, and C3 acts on C3aR in microglia to inhibit its phagocytosis of synapses, thereby reducing synaptic loss after sleep deprivation, improving cognitive decline related to sleep loss, which provides a novel mechanism underlying the role of DEX in alleviating cognitive deficits.
Zhai Qian, a doctoral student and assistant researcher from the FAH is the first author, and Professor Wang Qiang and Professor Ma Daqing are the corresponding authors. The FAH is the first affiliation of this article.
Full-text link:https://www.bjanaesthesia.org/article/S0007-0912(23)00281-7/fulltext