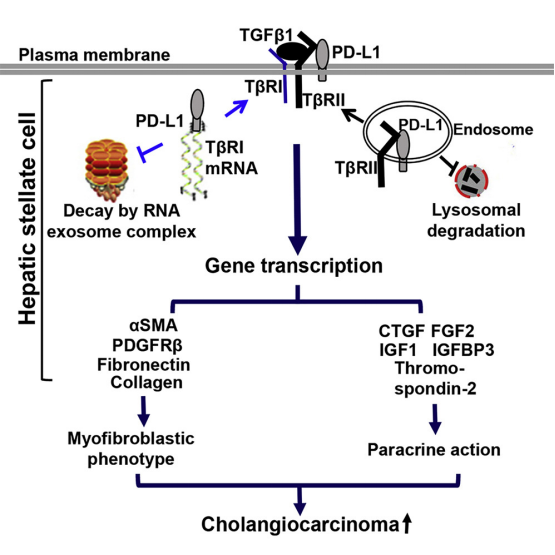
Intrahepatic cholangiocarcinoma is the second most common primary malignant tumor of the liver, merely secondly to hepatocellular carcinoma. The incidence and progression of intrahepatic cholangiocarcinoma is not only associated with the molecular events of tumor cells, but also regulated by the tumor microenvironment. The components of liver tumor microenvironment include cancer-associated fibroblasts, vascular endothelial cells, immune cells and extracellular matrix,etc. In healthy liver, hepatic stellate cells are at the rest state, whereas transform into cancer-associated fibroblasts when stimulated by tumor cells. TGF-β has been recognized as acytokine that effectively induces hepatic stellate cells to differentiate intocancer-associated fibroblasts. The checkpoint molecule PD-L1 is usually expressed in tumor cells and immune cells, while PD-1, the receptor of PD-L1, is mainly expressed in T cells. Monoclonal antibody specific to PD-1 and PD-L1 can block the interaction between PD-L1 and PD-1, thereby enhancing the anti-tumor capability of immune cells. PD-L1/PD-1 immunotherapy has broad prospects for clinical application. Nevertheless, the expression and function of PD-L1 in hepatic stellate cells remain elusive.
Recently, the research team led by Professor Liu Qingguang from Department of Hepatobiliary Surgery of the First Affiliated Hospital of Xi 'an Jiaotong University (XJTU), cooperated with the team of Professor Ningling Kang from Hormel Institute of University of Minnesota, and the team led by Professor Vijay H. Shah from GI Research Unit of Mayo Clinic to innovatively find that PD-L1 can promote the transformation of hepatic stellate cells into cancer-associated fibroblasts by regulating the TGF-β receptor I (TβRI) and TβRII. PD-L1 can bind to TβRII protein, which can protect TβRII from lysosome-mediated degradation. More interestingly, PD-L1 can also bind to TβRI mRNA, thereby protecting TβRI mRNA from degradation by RNA exosome complex. RNA-sequencing demonstrated that PD-L1 promoted hepatic stellate cells to express the marker molecules of cancer-associated fibroblasts and multiple tumor-promoting factors. In addition, transgenic mice with targeting PD-L1 knockdown hepatic stellate cells were also established in this study. By implanting cholangiocarcinoma cells into the liver of these mice, the growth of cholangiocarcinoma in the mouse liver was significantly constrained after targeting PD-L1 knockdown in hepatic stellate cells.

Recently, relevant research results were published as an Article entitled PD-L1 promotes myofibroblastic activation of hepatic stellate cells by distinct mechanisms selective for TGF-βreceptor I versus IIinCell Reports, a global authoritative journal in the field of cell biology, with an impact factor of 9.423 and 5-year impact factor of 10.394. Doctoral student Sun Liankang from Department of Hepatobiliary Surgery of our hospital is the first author, Professor Liu Qingguang and Associate Professor Tu Kangsheng are participating authors, and Professor Vijay H. Shah and Professor Ningling Kang are co-corresponding authors of this article. In recent years, Professor Liu Qingguang's team has been devoted to the basic-clinical-translational research and discipline construction of tumor microenvironment, emphasized the training and cultivation of postgraduates' clinical and scientific thinking, adopted the training mode of clinical practice followed by basic research, and achieved a series of scientific research achievements with clinical application prospects.
Article link:https://www.sciencedirect.com/science/article/pii/S2211124722000651?via%3Dihub