DNA replication is the foundation of biological inheritance and species uniqueness. In all forms of DNA replication, highly-conserved DNA polymerase is necessary to accomplish DNA replication. Because DNA polymerase needs to slide rapidly on DNA double helix, its cyclic β subunit provides the possibility of sliding. Therefore, the process of DNA replication is implemented surrounding the loading and unloading of β subunit of DNA polymerase onto DNA, which is the processivity-promoting factor during DNA replication process. DNA double helix completes the replication by rapidly sliding on the β subunit (Figure 1).
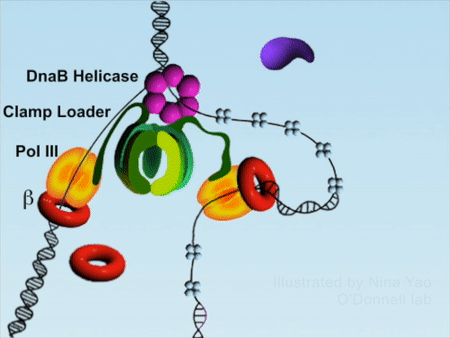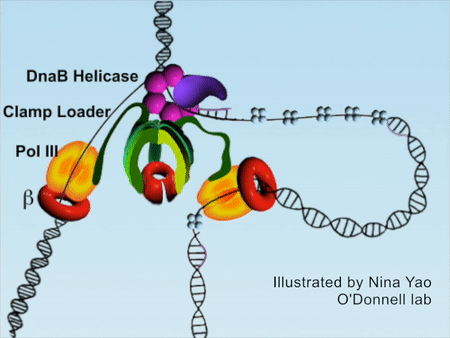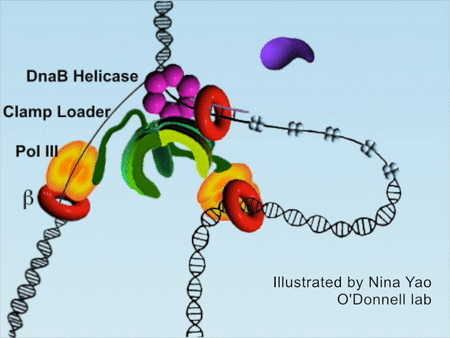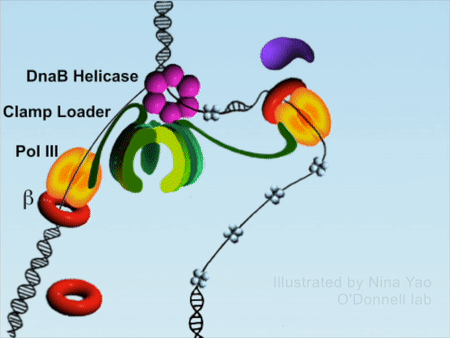
Figure 1DNA replication process (red color denotes β subunit).
Meantime, due to the critical role of DNA polymerase β subunit in bacterial replication and tumorigenesis, the research and development of its inhibitors have captivated widespread attention. Multiple types of DNA polymerase β subunit inhibitors are undergoing different stages of research and development, and certain small-molecule inhibitors have been approved as anti-tumor drugs by FDA. It has been reported that previous published inhibitors (including small molecules and polypeptides) and β subunit-binding proteins all target the hydrophobic protein binding pocket on the β-clamp.

The team led by Liu Bing from BioBank of the First Affiliated Hospital of Xi’an Jiaotong University (XJTU) investigated phage, the natural killer of bacteria and found that phage polypeptide Gp168 can inhibit the synthesis of bacterial DNA, and analyzed the complex structure of Gp168 and DNA polymerase β subunit by cryogenic electron microscopy, unraveling an alternative mechanism for Gp168 to inhibit the synthesis of bacterial DNA. On October 7, 2021, relevant research results were published in the globally top-notched journalNucleic Acids Research(IF=16.97). They proposed that Gp168 is the first bacteriophage β subunit inhibitor, the first natural β subunit inhibitor as well as the first molecule that does not target the hydrophobic pocket of β-clamp. Besides, theprotein only contains twoαhelices, which belongs to the category of polypeptide drugs. This polypeptide has been confirmed toexert broad-spectrum antibacterial effect. Consequently, our findings provide a novel strategy to combat bacterial multi-drug resistance, and relevant clinical trials are currently being prepared.
(https://aca demic.oup.com/nar/advance-article/doi/10.1093/nar/gkab875/6382387?login=true)
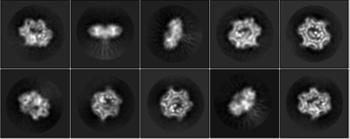
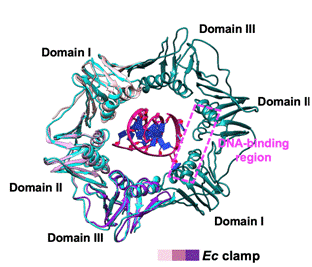
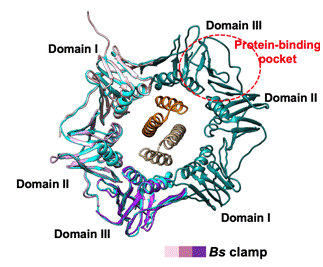
Figure 2 Reference-free 2D class average of cryogenic electron microscopy (top)
β subunit binds to DNA (middle), and DNA in β subunit is replaced by Gp168 (down)
Bruker 600MHz NMR spectrometer and TALOS cryogenic electron microscope from XJTU Instrument Sharing Center provide valuable opportunity for structural analysis. Professor Liu Bing from BioBank of our hospital is the corresponding author, Professor Wang Yawen is the co-corresponding author, and Liu Yang, a postgraduate student of our hospital, is the co-first author. Our hospital is the first and corresponding affiliation. Professor Zhang Lei from School of Physics of XJTU and Professor Wang Hongliang from Department of Pathogen Biology and Immunology of XJTU Health Science Center are co-authors of this article.
