On March 31, 2020, the team led by Professor Ma Xiancang from the First Affiliated Hospital of Xi 'an Jiaotong University (XJTU) published another online Article entitled Metagenome-wide association of gut microbiome features for schizophrenia in Nature Communications, which reported novel research results on the mechanism of gut-brain axis in schizophrenia.

This is the first study to perform metagenomic data analysis in medication-free schizophrenia patients worldwide. A variety of bacterial species associated with schizophrenia were identified. Transplantation of a schizophrenia-enriched bacterium could induce deficits in social behaviors and alter neurotransmitter levels in peripheral tissues in the recipient mice.
This study was jointly accomplished by the team lead by Professor Ma Xiancang from Department of Psychiatry of our hospital and the team pioneered by Research Fellow Jia Huijue from BGI-Shenzhen (BGI). The First Affiliated Hospital of XJTU is the first affiliation of this article. Dr. Zhu Feng from our hospital is the first author, Dr. Wang Wei from our hospital, Dr. Guo Ruijin, Dr. Ju Yanmei and Dr. Wang Qi from BGI are the co-first authors due to equal contribution. Professor Ma Xiancang is the corresponding author, Research Fellow Jia Huijue and Professor Karsten Kristiansen from BGI are the co-corresponding authors.
In August 2019, Professor Ma Xiancang's team published an article in Molecular Psychiatry, a top-notch international journal in the field of psychiatry, which for the first time revealed that gut microbiota from schizophrenic patients can regulate the tryptophan-kynurenine metabolic pathway and induce schizophrenia-related behaviors in mouse models.
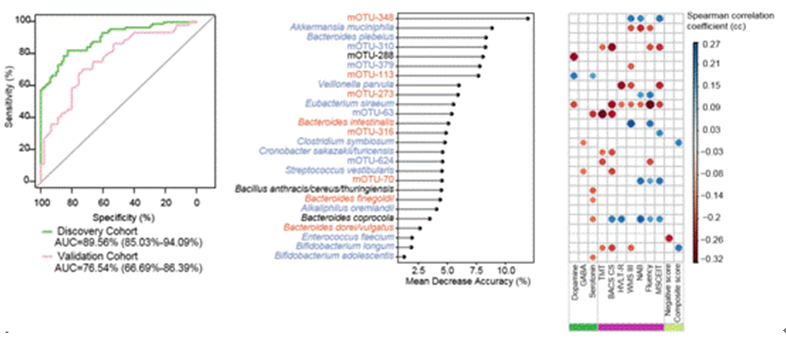
To further confirm schizophrenic patients presenting with gut microbial disorders, in this study, fecal microbiome based on 90 medication-free schizophrenic patients and 81 controls was analyzed by shotgun metagenome sequencing, and 83 bacterial species were identified to be associated with schizophrenia. The random forest model was utilized to demonstrate that 26 bacterial species can effectively distinguish schizophrenic patients from controls with an area under the receiver operating characteristic curve (AUC) of 0.896, which replicated the microbiome-based disease classifier in a cohort study of 45 patients and 45 controls. These findings suggest that gut microbiota may serve as a biomarker for the diagnosis of schizophrenia. The results of gut-brain module (GBMs) suggest that the functional neuroactive potentials of schizophrenia-associated bacterial species include short-chain fatty acid synthesis, tryptophan metabolism and synthesis/degradation of several neurotransmitters (glutamate, GABA and nitric oxide,etc.).
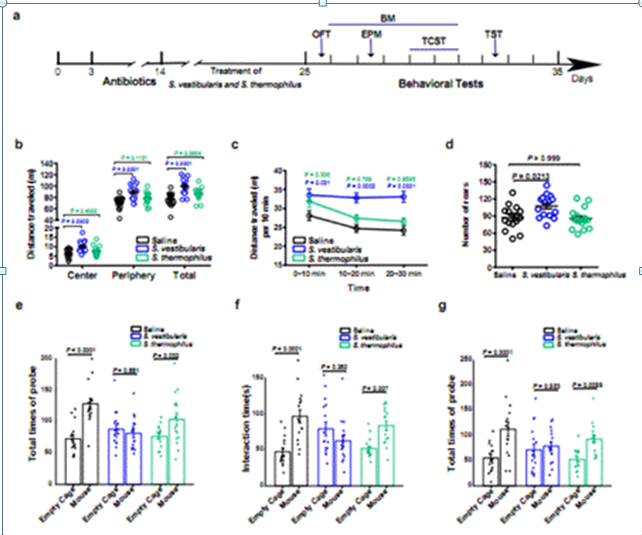
Streptococcus vestibularis is a type of schizophrenia-enriched bacterium, which not only participates in the glutamate synthesis and GABA degradation, but also has a significantly negative correlation with the score of Brief Assessment of Cognition in Schizophrenia (BACS). Compared to the control mice gavaged with saline and Streptococcus thermophilus, the Streptococcus vestibularis-treated mice exhibited an increase in the psychomotor excitability and social disorder, accompanied by neurotransmitter changes including a decrease in dopamine and GABA in the peripheral tissues and an increase in 5-HT, and up-regulated the expression of various inflammation-related genes in the intestine. In conclusion, this study identified a number of schizophrenia-associated bacterial species representing potential microbial targets for future treatment and emphasized the likely importance of microbial metabolites affecting the development of schizophrenia.
Article link: https://www.nature.com/articles/s41467-020-15457-9