Recently, research from Professor Ren Juan’s team in the Department of Radiotherapy of the First Affiliated Hospital (FAH) of Xi’an Jiaotong University (XJTU), entitled “GPR4 promotes immune exclusion in colon cancer through LOXL2-mediated extracellular matrix remodeling”, was published online in the Nature Research journal Nature Communications (CAS Q1; Impact Factor:15.7). PhD candidate Bai Shuheng is the sole first author, Professor Ren Juan is the sole corresponding author, and the FAH of XJTU is the sole institution that completed the study.

Tumor immune exclusion is a major cause of immunotherapy failure, and the physical barrier formed by the extracellular matrix is a key mediating factor. It is known that tumor hypoxia and an acidic microenvironment exacerbate this phenomenon, yet the intrinsic regulatory mechanisms remain unclear. Professor Ren Juan’s team conducted a systematic investigation into “how an acidic microenvironment drives tumor immune exclusion via proton-sensing receptors”. Using advanced techniques such as second-harmonic generation imaging and multiplex immunofluorescence, the team achieved quantitative characterization of extracellular matrix remodeling.
The study revealed that the proton-sensing G protein-coupled receptor GPR4 exerts dual effects in colon cancer through the JAK2/STAT3 signaling pathway: on the one hand, it regulates LOXL2 to promote collagen fiber alignment; on the other hand, it modulates TGF-β to enhance type I collagen expression. Together, these actions drive extracellular matrix structural remodeling and deposition, creating an immune-excluded tumor microenvironment and impeding immune cell infiltration.
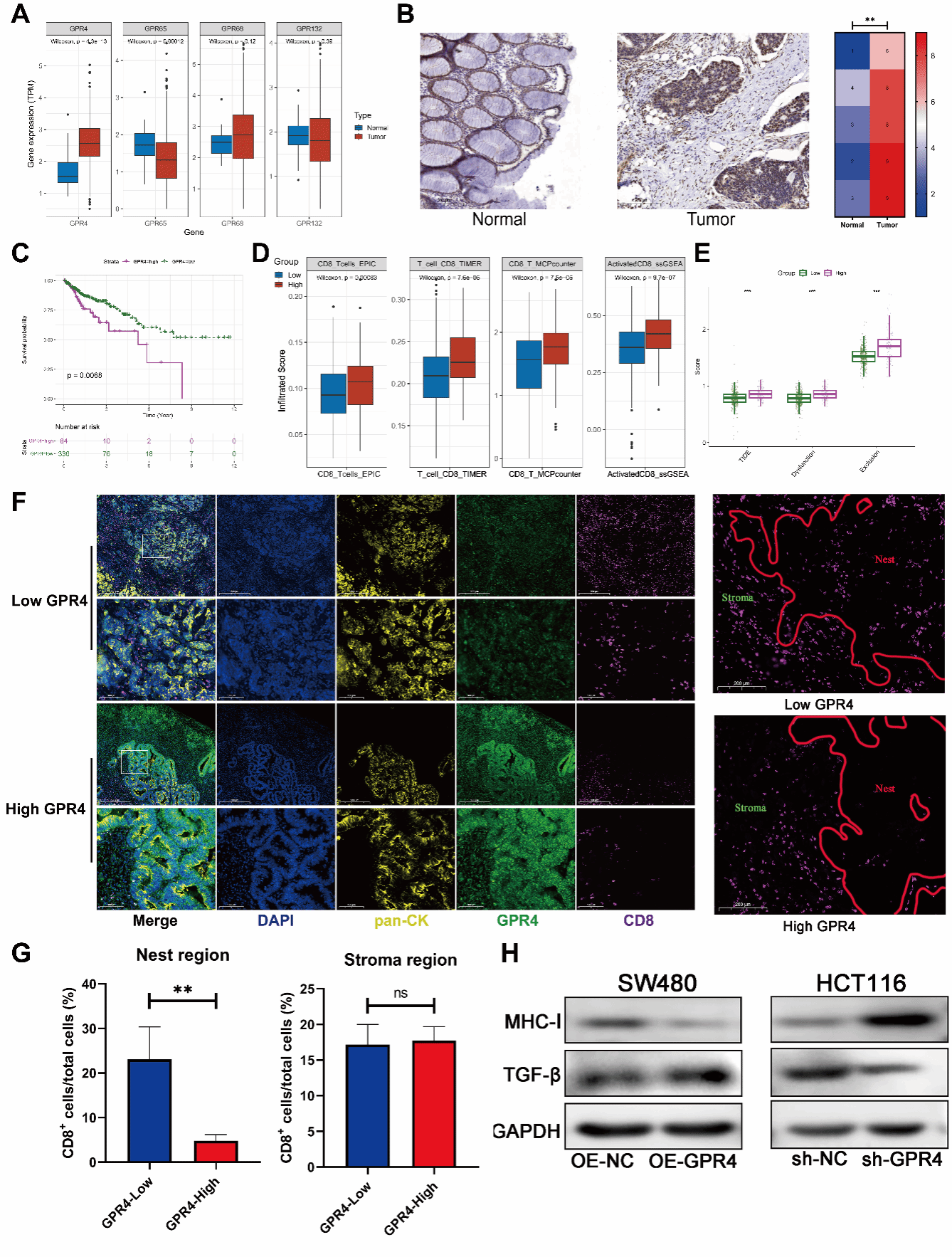
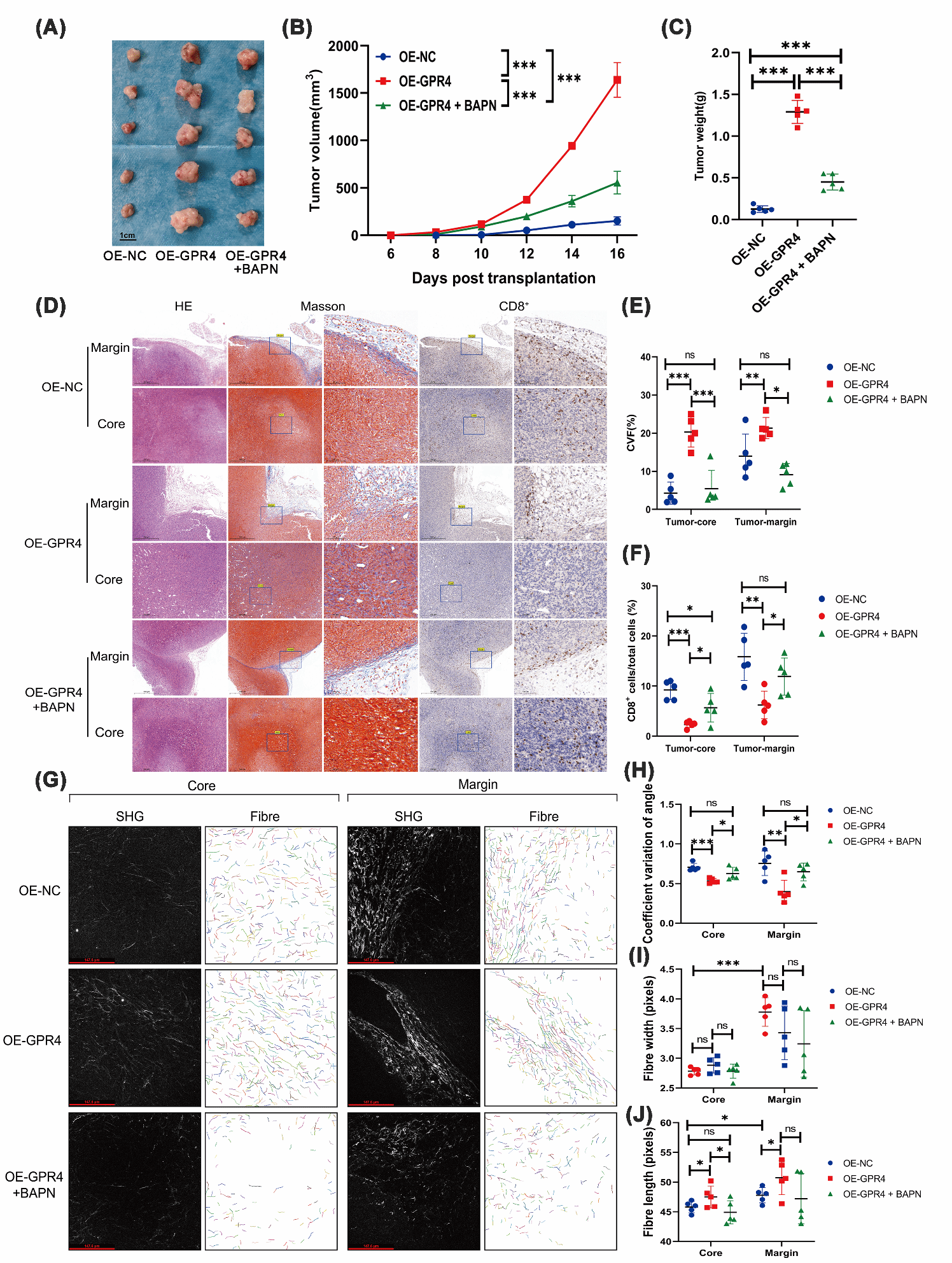
Based on this mechanism, the team developed a targeted intervention strategy: inhibiting the JAK2/STAT3 pathway or blocking LOXL2 function can effectively reverse tumor immune exclusion and significantly enhance responses to immunotherapy, an effect that has been validated in animal models.
This study deepens understanding of the complex relationship between the tumor microenvironment and immune regulation and has important clinical translational value. GPR4 and its downstream signaling molecules may serve as biomarkers for predicting immunotherapy efficacy and provide new strategies for combination targeted interventions. These findings are expected to help overcome immunotherapy resistance in colon cancer and other solid tumors and to provide a new targeting direction for the development of related immunotherapeutic drugs.