On December 9, 2025, Professor Guo Shiwen’s team from the Department of Neurosurgery of the First Affiliated Hospital (FAH) of Xi’an Jiaotong University (XJTU), in collaboration with partner institutions, published an original research article in the international journal Cancer Letters (CAS Division 1; JCR Q1; impact factor =10.1). The study reveals a novel molecular mechanism whereby hypoxic brain tumor stem cells (BTSCs) drive the progression of glioma by transferring “nuclear activating microRNA” (NamiRNA) via exosomes. The work also validated the efficacy of a potential targeted drug, providing a new theoretical basis for precision interventions in glioma.
Glioblastoma (GBM) is the most aggressive primary brain tumor, posing major therapeutic challenges. Epidemiological data show that the median survival of patients is less than 15 months, and more than 70% of patients relapse within one year after standard treatment, with the presence of BTSCs being a key underlying cause. These cells possess strong self-renewal and differentiation capacities; they can persist after treatment and drive tumor regrowth, and the hypoxic state of the tumor microenvironment further amplifies their malignant biological behavior.
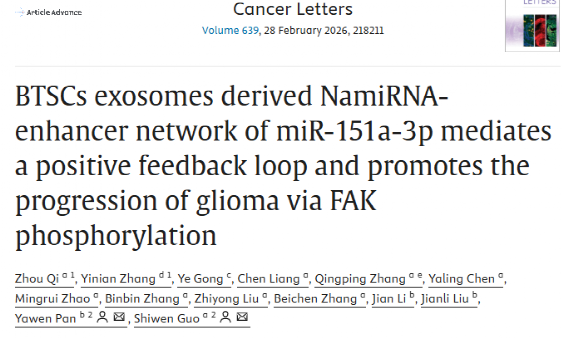
The research team conducted a systematic investigation into the core mechanism by which exosomes derived from hypoxic BTSCs regulate glioma progression. High-throughput sequencing revealed that miR-151a-3p is significantly enriched in exosomes from hypoxic BTSCs. This miRNA is a novel NamiRNA that can enter the nucleus to exert regulatory functions, differing from the conventional functional paradigm of cytoplasmic microRNAs that suppress target gene expression. Mechanistic studies showed that, within the nucleus, miR-151a-3p specifically binds to the enhancer region of the PDE4D gene and initiates transcription. The upregulated PDE4D protein interacts with focal adhesion kinase (FAK), activates the YAP signaling pathway, and promotes glioma cell proliferation and invasion. Meanwhile, activation of PDE4D signaling in turn enhances miR-151a-3p expression, forming a self-reinforcing positive feedback loop that drives sustained tumor progression.
Using molecular docking, the team identified the approved MET inhibitor capmatinib. Animal experiments confirmed favorable in vivo safety, with no obvious pathological damage observed in major organs.
This study is the first to systematically elucidate the complete mechanism by which hypoxic BTSC-derived exosomes drive the progression of glioma through a “NamiRNA enhancer network”. It provides a new theoretical perspective for understanding GBM recurrence and drug resistance, and lays the groundwork for the development of precision therapeutic strategies targeting BTSCs.
In this study, Professor Guo Shiwen and Professor Pan Yawen from the Second Hospital of Lanzhou University served as co-corresponding authors. The first authors are Dr. Qi Zhou from the Department of Neurosurgery and Professor Zhang Yinian from Zhujiang Hospital, Southern Medical University. The study was supported by projects such as the Shaanxi Provincial Key Research and Development Program.