At present, clinical prevention and treatment of venous thrombosis mainly rely on anticoagulant drugs and surgical thrombectomy. This dilemma of “drug-related risks and surgical invasiveness” has long limited the effectiveness of thrombosis prevention and control, and there is an urgent need for a safe, noninvasive and easily implementable new intervention strategy.
To address this clinical challenge, the Surgical DreamWorks team of the First Affiliated Hospital (FAH) of Xi’an Jiaotong University (XJTU) recently published a research article entitled “Magnetically-Induced Suppression of Oxidative Stress Prevents Venous Thrombosis” in the journal Advanced Science. The study, for the first time, systematically demonstrated that static magnetic fields (SMF) can effectively prevent venous thrombosis by inhibiting oxidative stress and protecting vascular endothelial cells. This breakthrough not only expands the scientific research frontier on the biological effects of magnetic fields, but also provides a completely new non-pharmacological, noninvasive strategy for the prevention of venous thrombosis.

This study innovatively established a mouse inferior vena cava thrombosis model by using FeCI₃ to induce oxidative stress injury in vascular endothelial cells, thereby simulating the pathological process of postoperative thrombosis caused by ischemia-reperfusion that is commonly seen in clinical surgery. Results following SMF intervention showed that moderate-intensity SMF (100-400 mT) can significantly reduce the incidence of thrombosis, decrease thrombus volume, improve blood flow perfusion and increase animal survival. Further investigations revealed that the specific mechanism involves SMF activating calcium ion channels, promoting intracellular ATP synthesis, thereby inhibiting NOX4 enzyme activity, reducing the generation of reactive oxygen species (ROS) such as hydrogen peroxide (H₂O₂), and effectively alleviating oxidative injury and apoptosis of vascular endothelial cells. The study, for the first time, clearly proposed that the “Ca⟡⁺-ATP-NOX4” signaling axis is the key pathway through which SMF exerts its vascular protective effects, filling a mechanistic gap in magnetic-field-based intervention for thrombosis and possessing important scientific value and clinical translational potential.
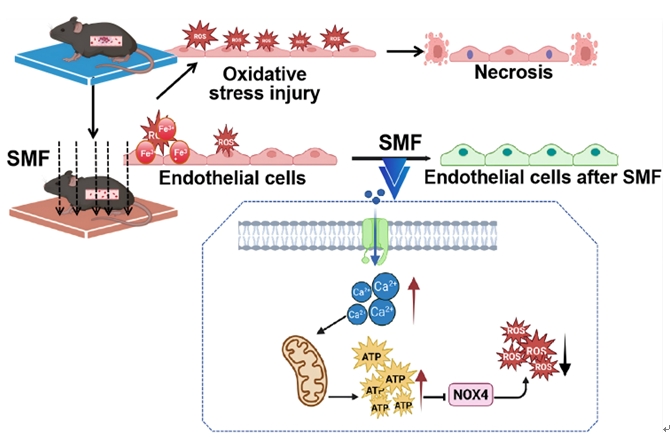
Under the guidance of Professor Lyu Yi, this study was independently completed by the Surgical DreamWorks team. Associate Research Fellow Zhang Nana and undergraduate student An Yirong are co-first authors, and Professor Lyu Yi and colleagues are co-corresponding authors. This work represents an important advance for the Surgical DreamWorks in the cross-disciplinary field of “physical-field intervention-biological response”, not only deepening the scientific connotation of medicine-engineering integration, but also laying a solid theoretical and experimental foundation for the future development of wearable magnetic-therapy devices and the establishment of personalized magnetic-field-based prevention and treatment systems.
Article link: https://doi.org/10.1002/advs.202513299