On December 6, 2025, Professor Yao Yu from the Department of Medical Oncology of the First Affiliated Hospital (FAH) of Xi’an Jiaotong University (XJTU), as a co-first author, published important findings in the international top-tier medical journal The Lancet Oncology (IF = 35.9), in a paper entitled “Vebreltinib in MET amplification-driven advanced non-small-cell lung cancer (KUNPENG): a single-arm, multi-cohort, multicentre, phase 2 study”. This study was led by President Wu Yilong of Guangdong Provincial People’s Hospital and completed in collaboration with multiple leading oncology teams across China, and for the first time globally confirmed the remarkable efficacy of vebreltinib, a highly selective MET-TKI independently developed in China.

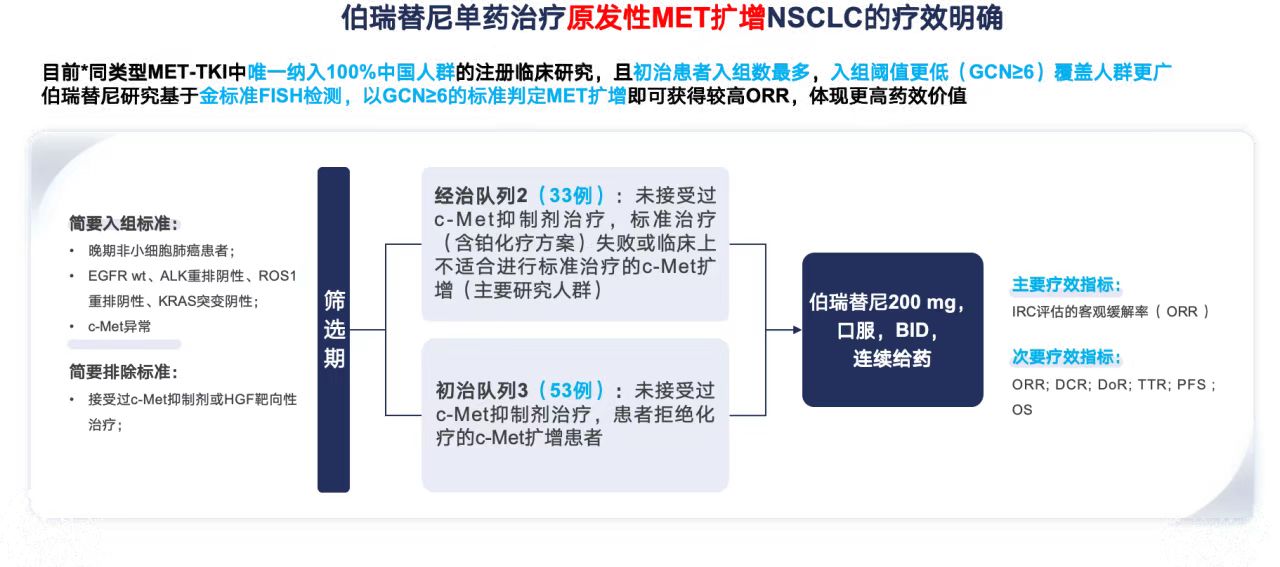
This study, named KUNPENG, is a prospective, multicenter, phase 2 pivotal clinical trial specifically targeting patients with the MET-amplified subtype of non-small-cell lung cancer (NSCLC). A total of 86 patients with locally advanced or metastatic disease confirmed by a central laboratory were enrolled (all were EGFR wild-type, ALK/ROS1 rearrangement-negative, KRAS mutation-negative, with a MET gene copy number ≥ 6), and all patients were included in both the full analysis set and the safety set for evaluation.
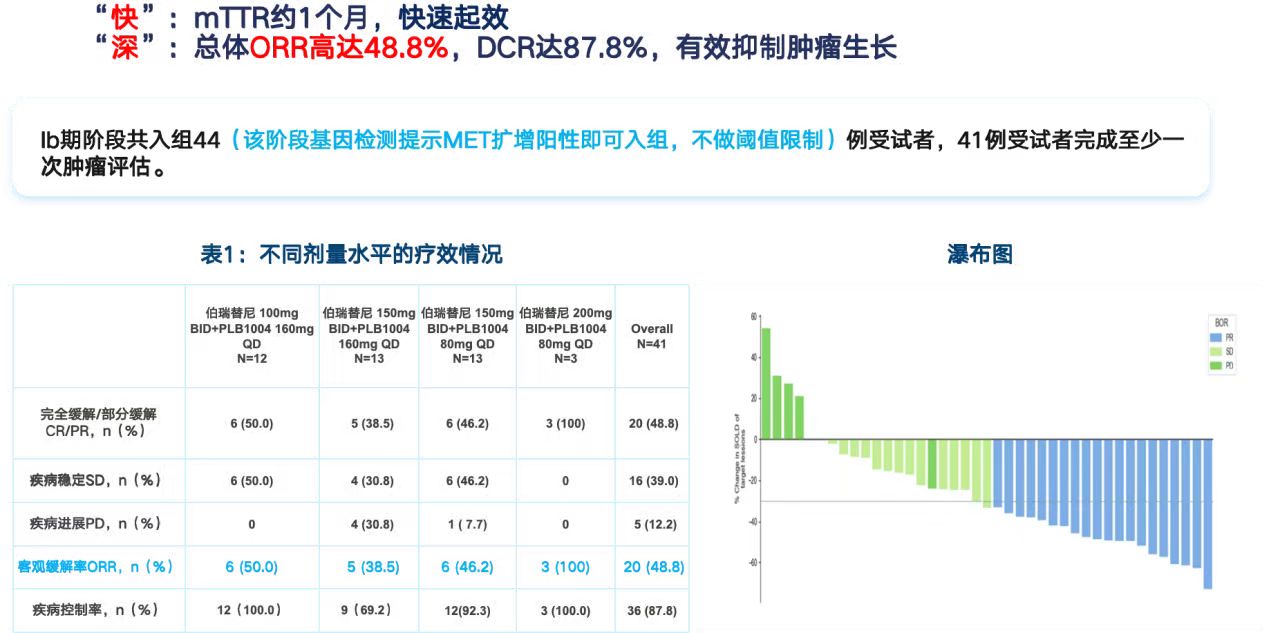
As of November 14, 2024, data from the independent blinded review center showed an overall objective response rate of 48.8%, a disease control rate of 77.9%, a median duration of response of 12.1 months, a median progression-free survival of 7.4 months, and a median overall survival of 13.9 months. The study demonstrated that, regardless of whether patients had received prior systemic therapy or had brain metastases, vebreltinib monotherapy produced significant and durable efficacy with a good safety profile, with the incidence of treatment-related adverse events of grade ≥3 being only 31.4%.

Based on these findings, vebreltinib was conditionally approved by the National Medical Products Administration on June 30, 2025, for the treatment of locally advanced or metastatic non-small-cell lung cancer with MET amplification, becoming the first highly selective MET-TKI approved in China for this indication. At the same time, this indication has been included in the 2025 edition of the National Reimbursement Drug List, enabling a broader patient population to benefit and marking a key breakthrough in China’s transition from a follower to a parallel runner in the field of MET-targeted therapy for lung cancer.
As a core participating team, Professor Yao Yu, together with team members Professor Tian Tao, Professor Liang Xuan, Professor Li Chunli, Dr. Fu Xiao and others, made outstanding contributions to key aspects such as study protocol design, patient enrollment, clinical trial implementation and data analysis. This fully demonstrates the professional strength of the Department of Medical Oncology of the FAH in the field of clinical oncology research and provides strong support for the development of precision therapy for lung cancer in China.
