Recently, a research team led by Professor Liu Bing from the First Affiliated Hospital (FAH) of Xi’an Jiaotong University (XJTU) published their findings as a cover article in Advanced Science, proposing a completely new strategy for antibiotic R&D. By drawing on the natural antibacterial mechanisms of bacteriophages, they successfully developed small-molecule candidate drugs targeting bacterial nucleoid-associated protein HU, cinnamic-hydroxamic-acid derivatives (CHADs), opening up a new pathway to address the global challenge of antibiotic resistance.

At present, antibiotic resistance has become a severe public health challenge. Owing to the intense selective pressure from long-term antibiotic use, resistance to traditional drug targets has increased sharply, making the development of antibacterial agents with entirely new mechanisms of action an urgent priority. The bacterial nucleoid-associated protein HU is highly conserved among pathogenic bacteria and is involved in core biological processes such as genome stability and biofilm formation; however, because it lacks typical enzymatic activity, it has long been regarded as a difficult-to-drug target.
Professor Liu Bing’s team previously discovered that the Gp46 protein of bacteriophage SPO1 can bind to HU and block its interaction with DNA, thereby achieving a form of “structural bactericidal” activity. Based on this mechanism, the team leveraged Huawei’s “Pangu” Drug Molecule Model for virtual screening, and from thousands of compounds, identified CHADs that can mimic the function of Gp46, followed by systematic validation of their activity.
The study demonstrated that CHADs directly inhibit the binding of HU to DNA, thereby disrupting the three-dimensional architecture of the bacterial genome and exhibiting a distinct “structural bactericidal” mode of action. At the same time, they reduce DNA supercoiling in a non-topoisomerase-dependent manner, reprogram bacterial gene pathways and weaken the ability of bacteria to adapt to environmental stresses. In addition, CHADs can inhibit biofilm formation and disrupt mature biofilm structures, and they are much less likely to induce resistance, with a resistance frequency as low as ~10-10, markedly outperforming traditional antibiotics.
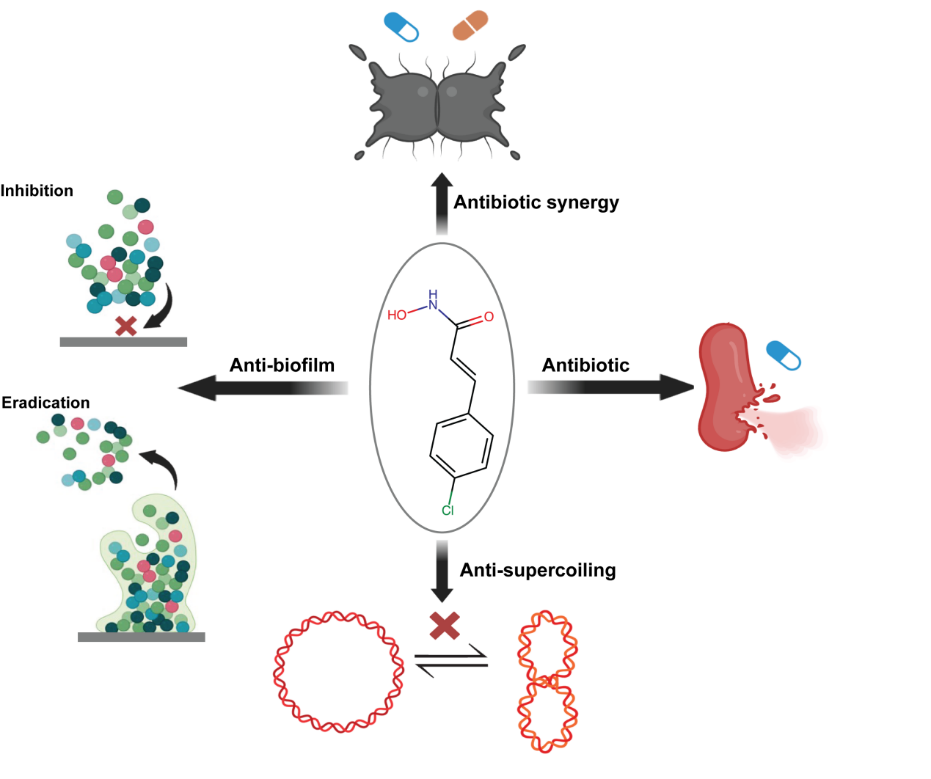
Trials showed that CHADs exhibit broad-spectrum antibacterial activity against both Gram-positive and Gram-negative pathogens, including Staphylococcus aureus, Acinetobacter baumannii and Mycobacterium tuberculosis, with the candidate molecule R4Cl demonstrating antibacterial efficacy comparable to that of classical antibiotics. In animal trials, CHADs significantly reduced bacterial burden and improved survival in skin infection and sepsis models, and their safety was preliminarily verified, laying the foundation for subsequent optimization of their druggability.
In this study, the potential of the HU protein as a drug target was demonstrated for the first time, and an innovative R&D pathway of “natural antibacterial mechanism → AI-based screening → small-molecule translation” was established. The FAH of XJTU is the primary contributing institution for this work; doctoral candidate Chen Huan is the first author, and Professor Liu Bing serves as co-corresponding authors.
Original article link:
https://advanced.onlinelibrary.wiley.com/doi/10.1002/advs.202509876