Recently, a multidisciplinary team led by Professor Lyu Yi and Associate Researcher Liu Xiaofei from the Institute of Regenerative and Reconstructive Medicine, MED-X Research Institute of the First Affiliated Hospital (FAH) of Xi’an Jiaotong University (XJTU), together with Professor Shen Shaohua from the State Key Laboratory of Multiphase Flow in Power Engineering, has made a key advance in the field of non-noble-metal electrocatalytic chlorine evolution. The related research results, entitled “Entropy-guided discovery of denary trirutile antimonates for electrocatalytic chlorine evolution”, were published in the top energy journal Joule, providing a low-cost and highly efficient solution for fields such as chemical production and medical disinfection.

Chlorine gas and reactive chlorine species are core raw materials for the chemical industry and key disinfectants for medical sterilization and water treatment. Conventional chlorine-evolution catalysts rely on noble metals, which are expensive and scarce, constraining the green transition of related industries. To address this challenge, the team proposed an innovative atomic-level, entropy-guided materials discovery strategy, opening a new pathway for the development of non-noble-metal catalysts.
Starting from the single-component CoSb2O6 structure, the researchers used the DFT system to systematically calculate the formation energies and lattice compatibilities of various metal substitutions. They screened 10 metals, such as Mg, Ti and V, from the periodic table and constructed a high-entropy system (MSb2O6) in which multiple metals can stably coexist. With the aid of a graph neural network (GNN) combined with Monte Carlo sampling, the team accurately predicted the optimal composition from 105 possible configurations and revealed the core mechanism by which the multi-element disordered structure of high-entropy materials lowers the system’s free energy and optimizes the electronic distribution at active sites, pinpointing the Cu sites as the optimal active centers.
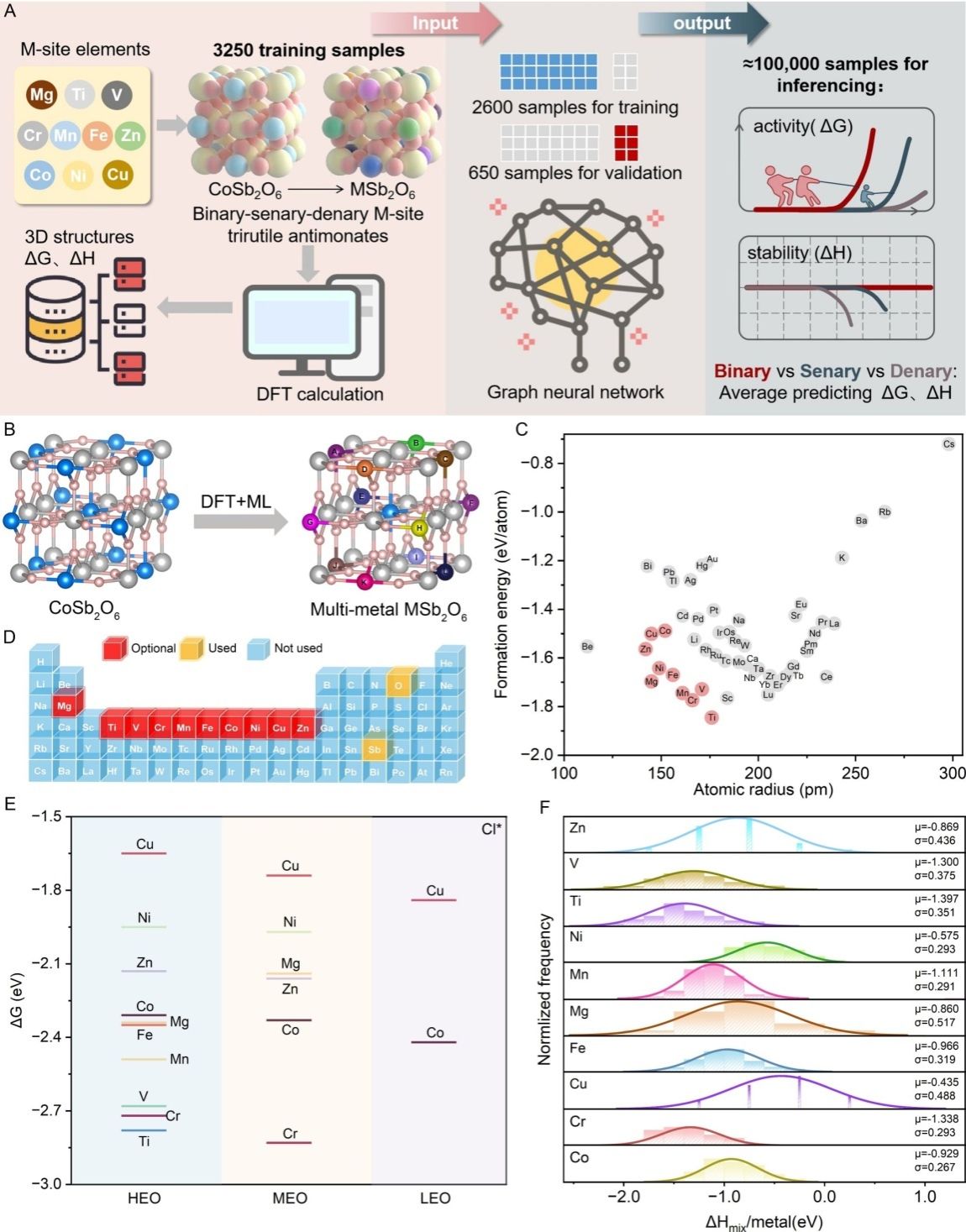
Using a solid-state calcination route at 1173 K, the team successfully synthesized a pure-phase denary high-entropy oxide, which was confirmed by XRD, HAADF-STEM and ICP-OES techniques to possess a homogeneous trirutile structure. In a 4 M NaCl-HCl electrolytic system, this catalyst exhibits outstanding performance: at a current density of 10 mA cm-2, the overpotential is only 24 mV, the Faradaic efficiency exceeds 95%, and it can operate stably for 160 hours without decay. Its mass activity reaches 78.1 A g-1, which is twice that of commercial DSA catalysts, while the cost is only 0.46 USD m-2, demonstrating extremely strong potential for industrial applications.
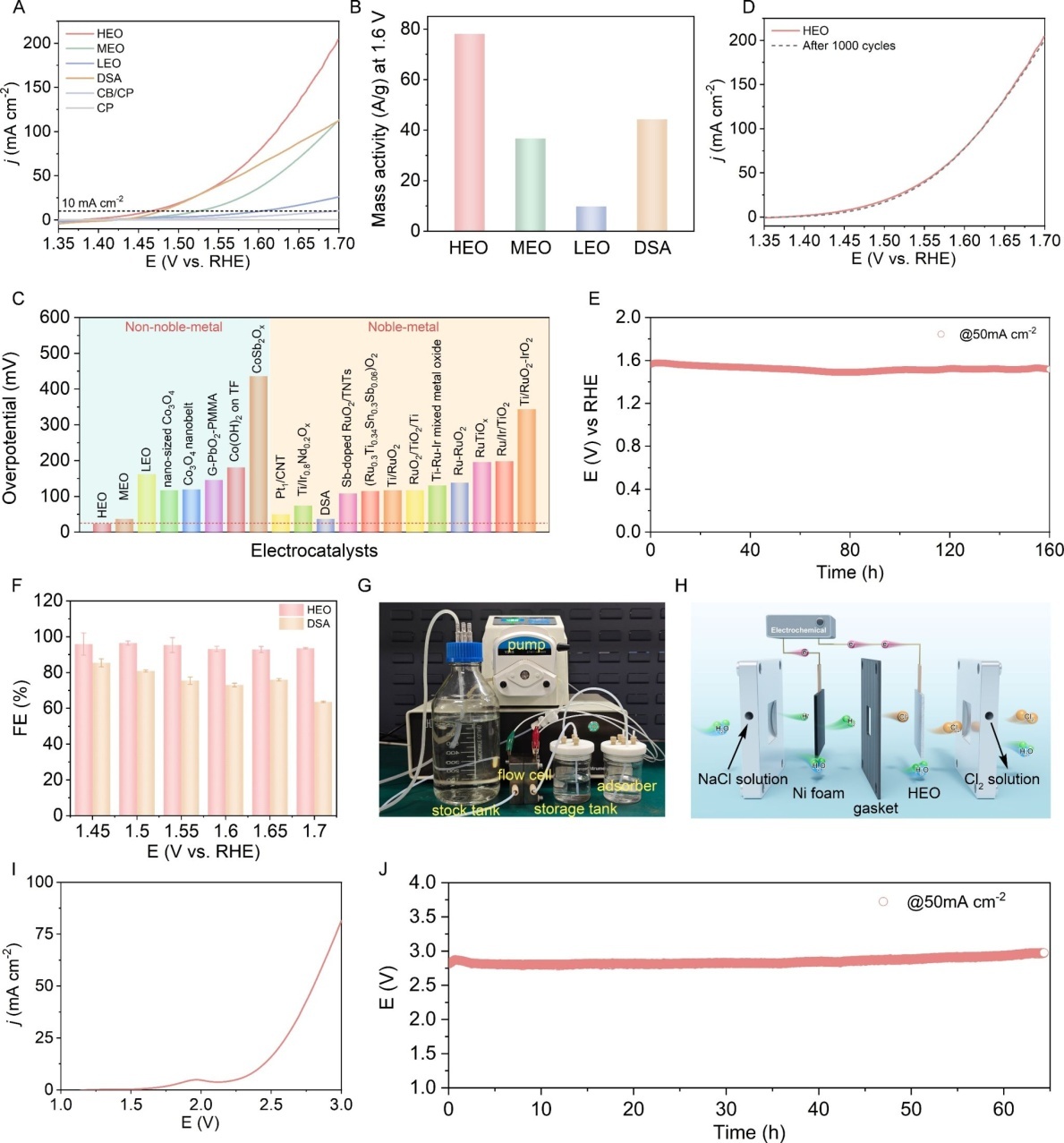
This study, through an “ML+DFT design” strategy, has achieved an innovative breakthrough in which multi-element disorder promotes the optimization of the electronic structure. The unveiled regulation mechanism of high-entropy materials lays the material and theoretical foundations for the development of novel electrocatalytic tumor therapies.
Article link:https://www.sciencedirect.com/science/article/pii/S2542435125003812