On October 19, 2025, Professor Yao Yu of the Department of Medical Oncology published an original research article in the New England Journal of Medicine (NEJM) entitled “Sacituzumab Tirumotecan in EGFR-TK1-Resistant, EGFR-Mutated Advanced NSCLC”. This research marks a major breakthrough in the post-TKI-resistance treatment landscape for EGFR-mutant non-small-cell lung cancer (NSCLC).
Lung cancer is a malignant tumor with the highest incidence and mortality rate in China. EGFR mutation is the most frequent driver gene among Asian patients with NSCLC. Although EGFR-tyrosine kinase inhibitors (EGFR-TKIs) are effective in initial treatment, the development of resistance is inevitable. Once patients acquire resistance to EGFR-TKIs, subsequent treatment options are limited and the efficacy remains unsatisfactory, which has long been a major difficulty and challenge in clinical practice.
OptiTROP-Lung04 is a multicenter, randomized, controlled trial conducted in China, designed to assess the efficacy and safety of the novel antibody-drug conjugate (ADC) Sacituzumab Tirumotecan (sac-TMT) versus standard platinum-based doublet chemotherapy in patients with EGFR-mutant advanced NSCLC after EGFR-TKI treatment failure. A total of 376 patients with EGFR-sensitizing mutations and locally advanced or metastatic non-squamous NSCLC who had progressed on prior EGFR-TKI therapy were enrolled. The patients were randomized 1:1 to receive sac-TMT monotherapy or pemetrexed plus platinum chemotherapy. The primary endpoint was progression-free survival (PFS), and the key secondary endpoint was overall survival (OS).
The results showed that PFS in the sac-TMT group was significantly superior to that in the chemotherapy group (Figure 1). The prespecified interim analysis of OS also met the positive threshold, with median OS not reached in the sac-TMT group versus 17.4 months in the chemotherapy group (HR, 0.60; two-sided P=0.001) (Figure 2). For objective response rate (ORR), the sac-TMT group achieved 60.6% versus 43.1% in the chemotherapy group; median duration of response (DoR) was 8.3 months and 4.2 months, respectively. The vast majority of prespecified subgroups, including patients who had received first- or second-line third-generation TKIs and those with brain or liver metastases, showed consistent trends of benefit.
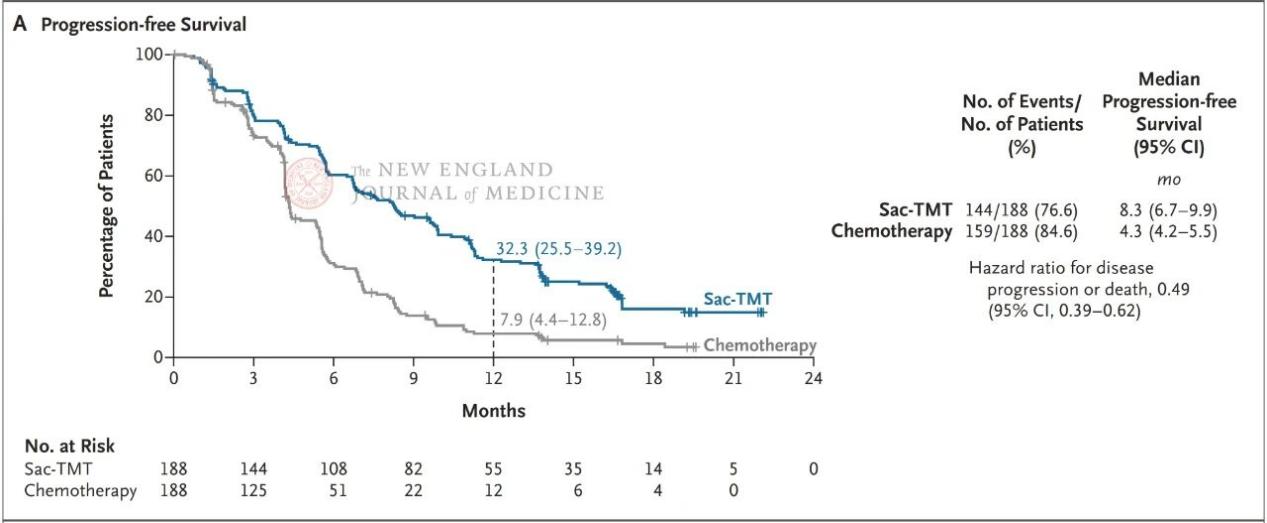
Figure 1. PFS analysis results for the sac-TMT group versus the chemotherapy group.
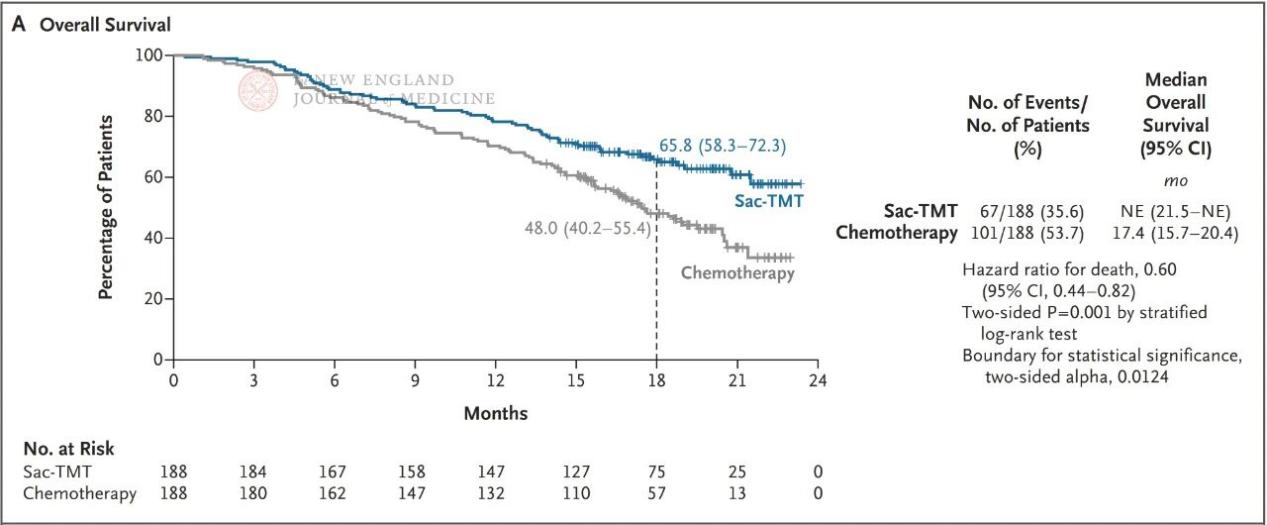
Figure 2. Interim OS analysis results for the sac-TMT group versus the chemotherapy group.
As a co-first author of the paper, Professor Yao Yu made outstanding contributions to key aspects of the study, including protocol design, patient enrollment, clinical conduct, and data analysis.
Article link: https://www.nejm.org/doi/full/10.1056/NEJMoa2512071