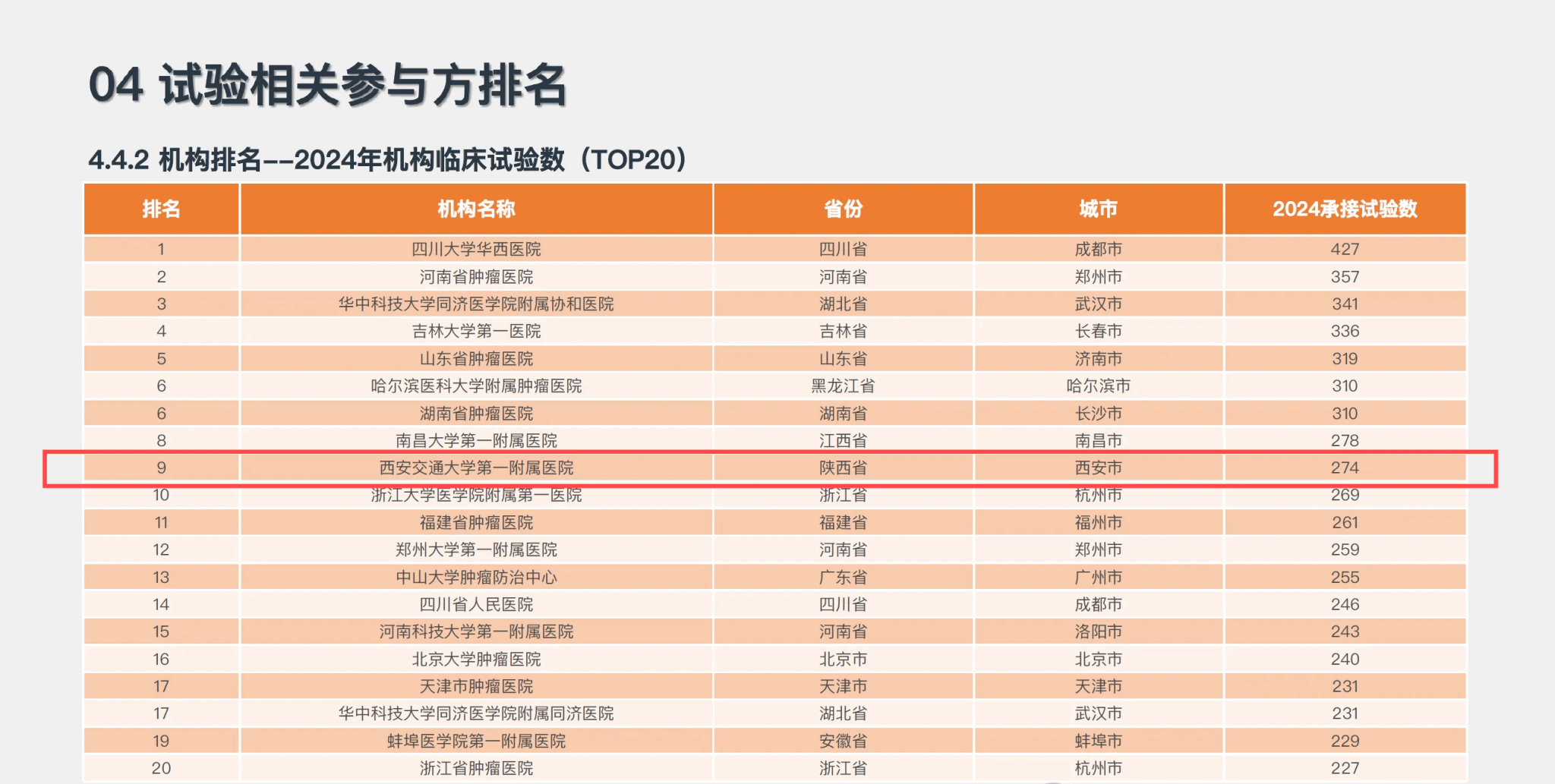
Recently, the Institution of Clinical Pharmacology released the “2024 National Report on Clinical Trials for Registered Drugs”. The clinical trial institution of our hospital ranked 9th in the 2024 National GCP (Good Clinical Practice) Institution Ranking, and secured the 1st position in the Northwest region, entering the national top ranks.
In recent years, China’s innovative pharmaceutical R&D has developed rapidly, with the quality of clinical trials reaching international advanced levels. As the final link in the pharmaceutical R&D process from basic research to clinical application, clinical trial institutions and their GCP platforms have received high attention from the National Medical Products Administration (NMPA) and the National Health Commission (NHC). Under the care and support of the hospital’s leadership, and with the collective efforts of all departments, the GCP Center of our hospital’s Department of Science and Technology has achieved remarkable progress. In 2024, our hospital undertook 360 new registered clinical trial projects (including new drugs, new medical devices, etc.), with over 1,000 ongoing projects. Guided by the management philosophy of "simplicity, efficiency, and compliance", the center has continuously optimized management processes, innovated operational models, and improved work efficiency. By integrating cutting-edge technologies and concepts into daily management practices, our hospital consistently advances the development of its high-quality GCP platform. During this critical period of building a National Medical Center with the collective efforts of the entire hospital, the GCP institution will remain committed to enhancing management efficiency and quality, safeguarding the safety of public medication, and contributing significantly to China’s innovative medical R&D.