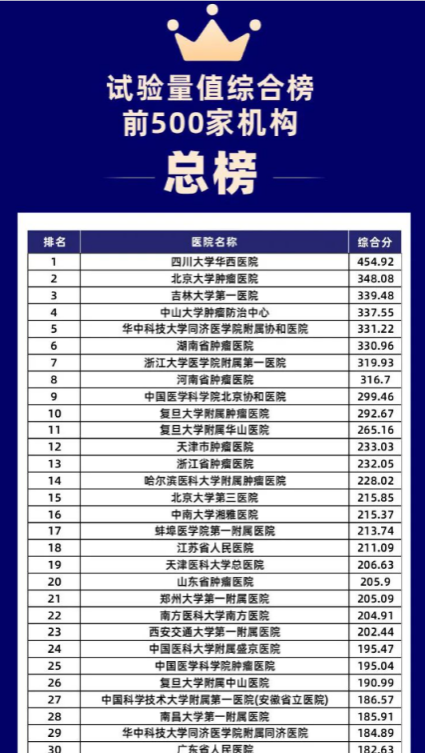
On March 17, 2023, the ranking list of Drug Clinical Trial Quantity in National GCP Institutions was grandly released. The First Affiliated Hospital (FAH) of Xi'an Jiaotong University (XJTU) ranked 23rd on the ranking list, moving up 6 spots from last year.
Under the guidance of expert working panel, the organizing committee of China Forums of Clinical Research Capacity Building and Human Research Participants Protection (CCHRPP) organized expert panel in the industry in combination with statistical analysis data from data companies within the industry to evaluate the ranking list of Drug Clinical Trial Quantity in National GCP Institutions. All data sources are obtained from "Drug Clinical Trial Registration and Information Publicity Platform" of CDE (http://www.chinadrugtrials.org.cn). The ranking list can reflect the support and promotion of drug clinical trial institutions for biomedical research and development to certain extent. The ranking list in 2023 compiled the implementation quantity of drug clinical trials from 2020 to 2022 by simultaneously considering the value indicators and weights of drug research and development, forming the new ranking list. This year, the data in 2019 were excluded and the data in 2022 were included in the ranking list to guarantee the presentation of data in recent three years, which can reflect latest dynamic development and evolution trend of the institutions.
The ranking list released in 2023 showcases new development and advancement of drug clinical trial quantity in our hospital over 2022. In 2022, Institution of Clinical Pharmacology of the FAH adhered to the development concept of "taking project quality as the core", focused upon National Medical Center and tertiary public hospital performance assessment indexes and took a series of management measures, such as promoting publicity, foreign exchange, strategic cooperation, recruiting projects, prioritizing project establishment, pre-review of ethical statement, prioritizing service and speeding up the review and signing of contracts, aiming to persistently improve the scale and quality of clinical trials in FAH. In 2022, the hospital undertook 239 clinical trial projects and received a total funding of 131 million Yuan, including 33 phase I drug clinical trials, an increase by 73.7% over 2021, 12 leading projects, an increase by 20.0% compared with 2021, and the quantity and quality of clinical trial projects have been equally enhanced. The quality of clinical trial projects has been highly recognized by relevant parties. In 2022, the hospital successfully passed national data verification and 6 roundsof multiple inspections in Shaanxi province.