Recently, Nucleic Acids Research published a research paper entitled "A temperature-driven DNA discrimination strategy to distinguish E.coli DNA and phage 5hmC-modified DNA", which systematically reveals a temperature-driven DNA discrimination mechanism and proposes a new paradigm of bacteriophage T4 exonuclease DexA and an uncharacterized Escherichia coli exonuclease as a rare pair of attack and defense duo arising from the same mechanism, which has important theoretical significance and potential application value.
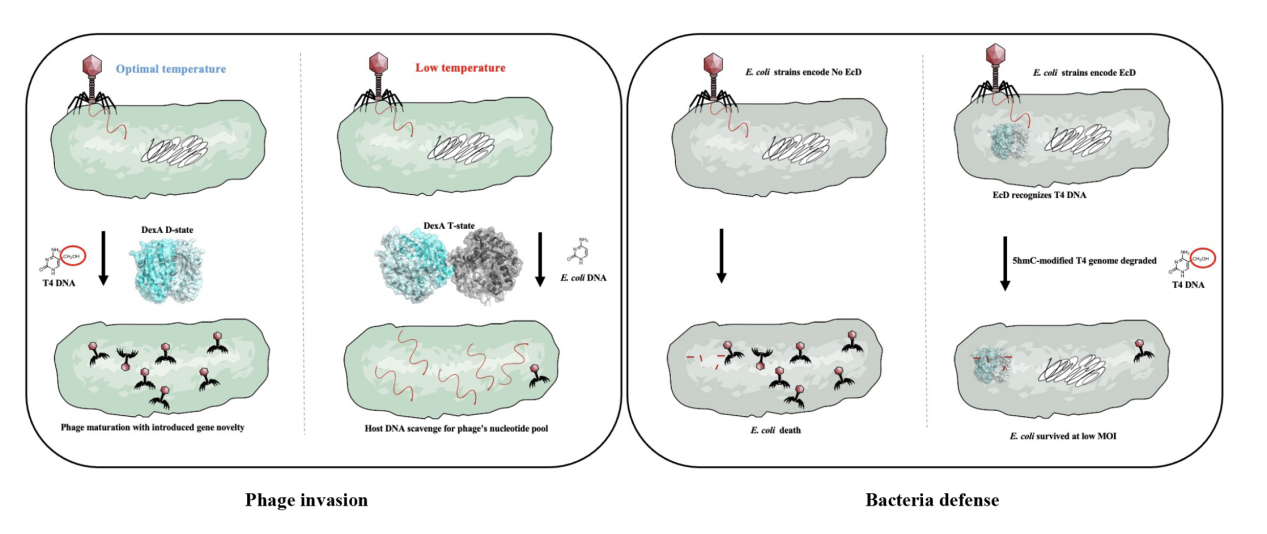
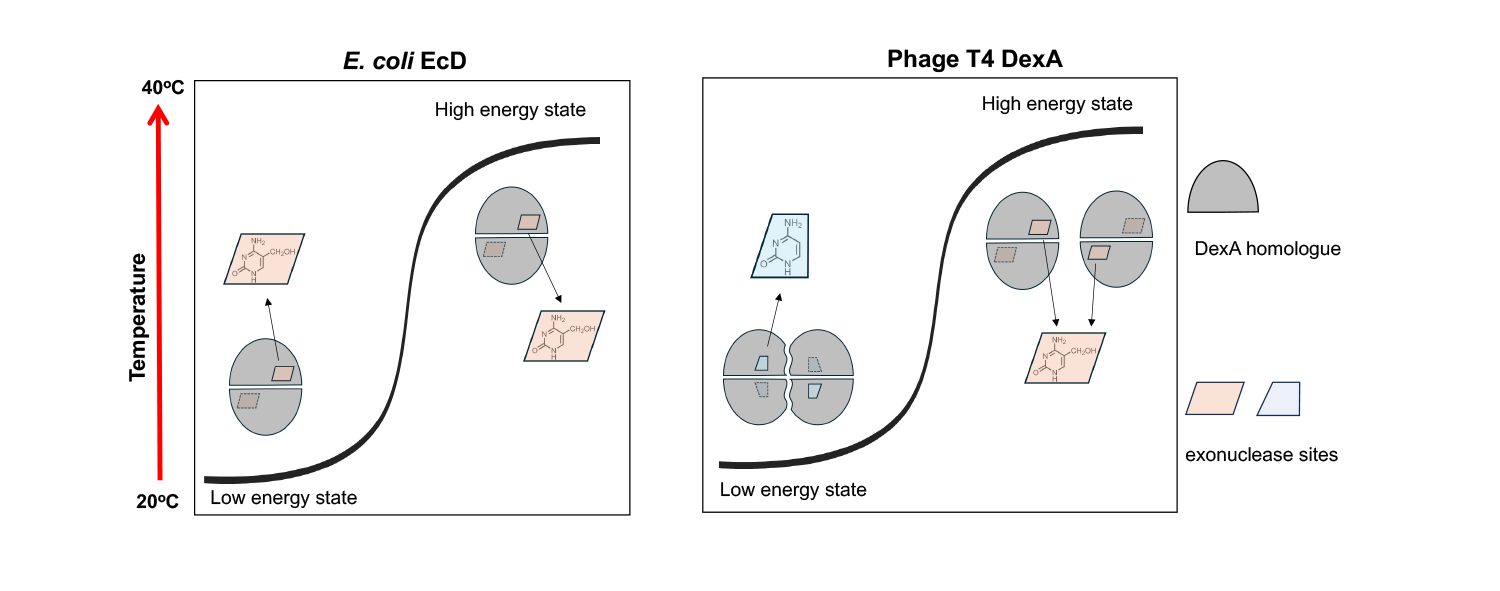
The authors reported that a temperature-driven quaternary fold switch between T4 bacteriophage-encoded DexA dimer and tetramer that facilitates cleavage of distinct DNA forms. At a low temperature (25°C), DexA is active in tetramer form responsible for scavenging unmodified E.coli DNA; At body temperature (37°C), DexA exists as a dimer, which specifically recognizes and cleaves 5- hydroxymethylcytosine (5hmC) using T4 DNA as the substrate and participates in genome recombination. This mechanism enables phage to flexibly realize dual functions of resource acquisition and gene regulation at different environmental temperatures.
This study further found that E.coli itself evolved a homologous exonuclease EcD of DexA, whose conformation is stable as a dimer, specifically targeting the phage DNA modified by 5hmC, thus forming a defense mechanism against T4 phage. This phenomenon of "host and virus share structural modules and perform opposing functions" reflects high-degree convergence and strategic antagonism during molecular evolution. Consequently, the research team proposed and defined the HREX system (HmC-Recognizing EXonuclease), which provided a novel cognitive framework for the "molecular arms race" between bacteria and phages.
This study was led by Professor Liu Bing from the First Affiliated Hospital (FAH) of Xi'an Jiaotong University (XJTU), and was supported by the National Key R&D Program of China, National Natural Science Foundation of China and Fundamental Research Funds for the Central Universities. NMR spectrometer was completed in the BioBank of FAH of XJTU.
This study not only deepens the understanding of the interaction mechanism between host and phage molecules, but also provides potential tools and theoretical support for establishing new strategies against phage pollution in microbial resource utilization, synthetic biology and fermentation industry in the future.